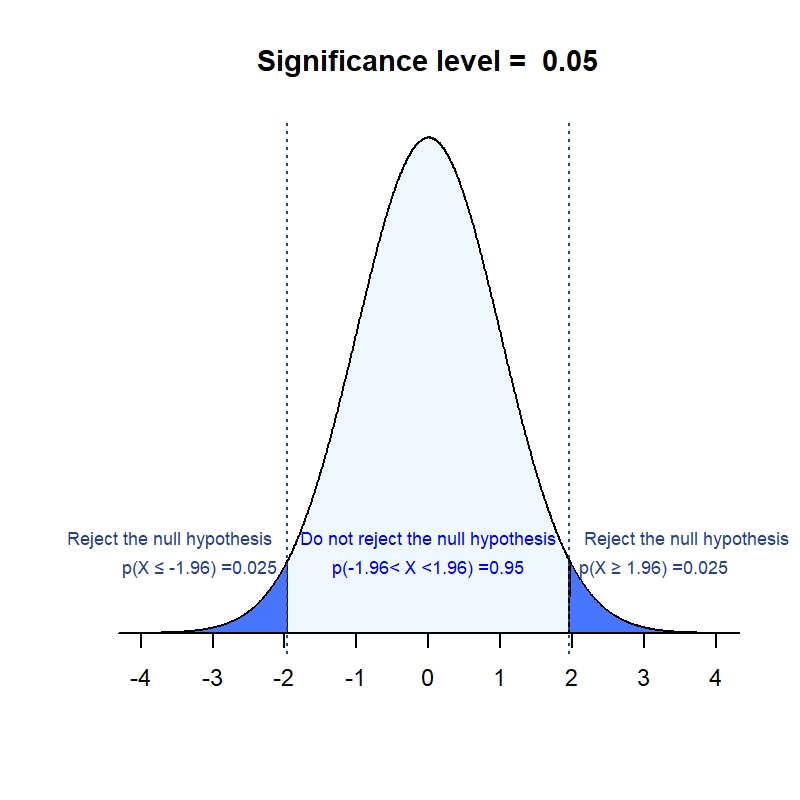
The first‐in‐human (FIH) clinical trial is an important milestone for each development program. For small (bio)pharma companies the FIH trial requires a significant investment, and every sponsor wants to make sure that all is well set for starting the trial. It is indisputable that successful execution of the FIH trials does require sponsors to coordinate the task with thorough consideration and planning across many disciplines. Especially, selection of the starting dose requires a cross‐functional collaboration, where preclinical knowledge needs to be transformed into clinical applications. This step could become a large hurdle which could even retard the progress of the program, if planned and strategic alignments are not reached within the project team as well as adequate actions are not taken well in advance of the FIH trial.

Over the past years, Venn Life Sciences as a consultant company, has been exposed to a significant number of FIH trials both in healthy volunteers as well as oncology patients. In this blog, we like to highlight several considerations, based on these experiences, that will need to be addressed for defining the starting dose and beyond in the FIH trial.
MABEL or NOAEL?
The EMA’s FIH guideline says, “Depending on the level of uncertainty regarding the human relevance of findings observed in nonclinical studies and the knowledge of the intended target, the starting dose should either be related to the MABEL (Minimum Anticipated Biological Effect Level), PAD (Pharmacologically Active Dose) or NOAEL (No Observed Adverse Effect Level).” Thus, the choice of MABEL- or NOAEL-based starting dose selection must be driven by overall assessments of available data and risk-based considerations. Typical factors for the assessments include but are not limited to:
It is highly recommended to make a rationalized choice of the MABEL- or NOAEL-based approach (e.g. using decision tree) with adequate documentation in investigator’s brochure, as this is a critical point of review by the competent authority/ethics committee.
Do you have sufficient/adequate data set for MABEL calculations?
To employ the MABEL-based starting dose establishment approach, it is essential that relevant data sets have been generated by the sponsor. Unlike toxicology (NOAEL)-based approach for FIH dose selection, which is rather straightforward, the type of data required for MABEL calculation can differ case by case (e.g. in vitro and/or in vivo data and in recent years increasingly involve modelling and simulation). Since generation of relevant data may take several months, any absence of relevant data for the MABEL calculation could cause a significant delay in the program. Therefore, it is pivotal that the project team plans this exercise way in advance of the FIH preparations.
How to define “minimum” effect?
One of the most frequently asked questions in terms of MABEL calculation is what degree of a biological response is considered “minimum”. Unfortunately, there is no standard answer for the question, and this is nowhere well-defined in the guidelines, as there are multiple unique factors that may need to be considered for each investigational product. For example, since receptor occupancy required for efficacy are expected to be different between antagonists and agonists even if the same receptor is targeted, different considerations are likely required for defining the MABELs for such cases. Ultimately, the sponsor is responsible to scientifically justify underlying rationale for defining the MABEL.
Starting dose calculation
While mechanistic state-of-the-art modelling (e.g. PK/PD and PBPK) is increasingly used in the calculation of the starting dose based on NOAEL, PAD and MABEL, it should be emphasized that the EMA guideline also refers to the application of allometric factors. Unless the drug product is associated with significant safety risk in human based on preclinical finding and/or a certain drug class (e.g. immune stimulator) and thus requires more sophisticated mathematical approach, empirical allometric scaling approach in combination with a simulation of exposure-pharmacological response relationship in human might also be sufficient for the starting dose selection for the FIH trial in healthy volunteers. Irrespective of approaches chosen, careful deliberation on the planning of the starting dose assessment is required, as unnecessary assessments during this process may also cause a delay in the program and a needless financial burden.
Applying safety factors
After the calculation of the MABEL, PAD, or NOAEL, additional safety factors are applied to account for several potential risks, such as the relevance of animal models, any uncertainties in the estimation of the MABEL, PAD, or NOAEL, or other unique safety or pharmacodynamic characteristics of the investigational product. Inadequate assessment of the safety factors may result in unnecessary risk for volunteers, or unnecessarily low doses in the first cohorts and as such additional dose escalation cohorts may be needed.
Be clever in trial design/protocol
Despite of all exceptional efforts and fancy mathematical MABEL calculations, pharmacokinetic properties might behave differently in human in the FIH trial, especially at the low starting dose, where PK tends to be non-linear. The clinical trial protocol, therefore, should clearly define the dose escalation rules to allow for the dose escalation to proceed in a scientifically, ethically as well as financially acceptable way. For example, the clinical protocol should include adequate language to allow for more aggressive dose increments, in case the observed exposure at the starting dose is significantly lower than predicted. Otherwise, volunteers will continue to be administered at dose levels even lower than the MABEL, which may result in the need of additional cohorts, or in the case of patients, treatment at an unnecessarily low and ineffective dose. We highly recommend authoring an adaptive FIH protocol, which will allow adjustments in the dosing schedule, circumventing delays in the trial execution.
Concluding remarks:

Tom Habraken (MSc) is Consultant Clinical Pharmacokinetics at Venn Life Sciences. He has over 6 years of experience in pharma/consultancy industry. He is an expert in Clinical Pharmacokinetics and has been involved in FIH trials in healthy volunteers as well as oncology patients. Tom is also an organizer and trainer of Venn’s Pharmacokinetics Course (LINK).

Marieke van den Dobbelsteen (PhD) is Senior Consultant Clinical Development as well as acting as Group Leader in Clinical Trial Management and Medical Writing Team at Venn Life Sciences. She has over 25 years of experience in pharma/consultancy industry. She is an expert in early drug development (early-clinical) and has ample experiences in managing as well as providing consulting for early clinical development including FIH trials.

Theo Reijmers (PhD) is Consultant Pharmacometrics at Venn Life Sciences. He has over 6 years of experience in pharma/consultancy industry (+ >10 years in academia). Theo is a pharmacometrician and has ample experience in PK/PD translational modelling in early-stage drug development (e.g. human dose prediction for first-in-human studies). He also has experiences with submitting modelling related output to FDA and EMA.

Katsuhiro Mihara (PhD) is Senior Consultant Clinical Development as well as acting as Head of Clinical Development Department at Venn Life Sciences. He has over 25 years of experience in pharma/consultancy industry. Katsu has a broad knowledge on early clinical development, clinical pharmacology as well as translational science and has been involved in numerous numbers of early-stage clinical projects as well as scientific due diligence projects for investors/pharma.
If you are interested to learn more about how to define a safe starting dose for your FIH trial, please contact Venn life Sciences at BD@vennlifesciences.com. We would welcome the opportunity to discuss efficient set up of your FIH trial and other related aspects.
Did you ever ask yourself one of the following questions?:
Statistics play a fundamental role at each step of a clinical trial (trial design, protocol development, the statistical analysis planification and execution, interpretation of the results). With the evolution of statistical science (e.g., progresses in the understanding of mechanisms generating missing data and methods to handle missing data) and the development of new clinical trial methodology concepts (e.g. adaptive designs, master protocols…) new challenges have emerged.

The recent adoption of the ICH E9 (R1) addendum on estimands and sensitivity analysis in clinical trials to the guideline on statistical principles for clinical trials and methods in 2020 reshaped the approach to study design and protocol development. An estimand is a precise description of the treatment effect to be estimated. The estimands have to be defined upfront, before considering the methods for answering the question (the statistical analysis itself). Clear study objectives and the scientific questions of interest have to be precisely described. Estimands consistent with the question of interest are then defined. Only after the estimands have been defined can the trial be adequately designed, ensuring all the data necessary to the estimands are collected. Finally, the statistical analysis methods can be chosen.
As a non-statistician clinical trial professional from the pharmaceutical industry, biotech industry or health research, you may feel uncomfortable with some statistical concepts or methods and in your interactions with statisticians.
At Venn Life Sciences Biometry Services, our expert methodology and biometry consultants provide training to fulfill this gap.
Several topics can be covered, and a training program specifically tailored to your own needs can be prepared:
Descriptive statistics
Inferential Statistics (Hypothesis tests)
Confidence intervals
Frequentist vs Bayesian inference
Type of variables
Measures of treatment effect
Choice of statistical test
Statistical modeling Sample-Size and Power
Design (parallel groups, crossover, factorial…)
Interventional/ non interventional studies
Bias reducing techniques (Randomisation Blinding)
Objectives, Endpoints
Estimands
Dose finding
Interim analyses
Adaptive designs
Master protocols (Basket; Umbrella, Platform- trials)
Diagnostic tests
Metanalysis Data Monitoring Committees
Analysis populations
Multiplicity
Superiority/Non inferiority, Equivalence
Handling of Missing data
Subgroup analyses
Interpretation of results
Reporting
Critical appraisal

The Food and Drug Administration (FDA) is an agency within the U.S. Department of Health and Human Services. It is the US Agency responsible for the regulation of drugs and biologics, including, vaccines for humans, blood and blood products and cellular and gene therapy products.
As the United States remains the largest pharmaceutical market in the world, any drug development program will require a close interaction with the FDA to ensure your development rationale is sound and that the design of your studies is fit-for-purpose.
Although a first contact with the FDA might sometimes feel intimidating, an early interaction will prove very valuable. Your first interaction with the FDA will likely be a pre-IND meeting. Sensu-stricto a pre-IND meeting is not required, it is however highly recommended to engage with the FDA in a pre-IND meeting for the following reasons:
Early Feedback from the FDA - The most valuable benefits of the pre-IND meeting are to receive early feedback directly from the FDA on your development program and to gain an understanding as to what the FDA’s expectations are for your drug.
Fine-Tuning of your Development Strategy – Taking the feedback in the FDA into account, offers you the opportunity to fine-tune your program development strategy, potentially saving time and money.
Relationship Building – The pre-IND meeting is also the opportunity to start building a working relationship with the FDA and individuals within the Agency that will be your contact persons.
In preparation of your pre-IND meeting, you will need to prepare a briefing package. In this briefing package you will provide background information on your compound and prepare the list of questions. When preparing these questions, there a few things you need to keep in mind:
• FDA meetings are most effective when they are focused on specific scientific or regulatory issues, such as clinical trial design, pharmacology studies, toxicology studies, acceptability of novel formulations, dosing limitations, data requirements for an IND application, etc.
• Speculative and open-ended questions are difficult to address; meetings are typically most productive when questions are focused and specific. Questions for the FDA should be posed in such a way that the agency can either agree or disagree with the question.
As the briefing package forms the basis of a successful meeting, it is important that it is well worked out. Taking advantage of the valuable input that can be gained from pre-IND meetings is essential for the further development of the drug and may reduce the drug’s time to market and ensure that the proposed studies are designed to provide useful information. Sponsors who are forthcoming with potential issues of concern during the drug development process will benefit from input provided by the regulatory agency.

Bruno Speder is currently VP, Regulatory Affairs & Consultancy Services at hVIVO & Venn Life Sciences, part of Open Orphan. He advises clients on the regulatory strategy of their vaccine development and supports them in their interactions with the Food and Drug Administration, European Medicines Agency and the national regulators in Europe. He holds a degree in Bioengineering from the University of Ghent, Belgium.
If you are interested to learn more about how our Regulatory Affairs department can support your development activities, please contact Venn life Sciences at getintouch@vennlife.com.
Design of Experiments (DoE), introduced in the 1920s, is a valuable modelling tool to support in amongst the systematic development of (bio)pharmaceutical products, processes and analytical methods. Especially in combination with risks assessments (e.g. FMEA), DoE is paramount of importance to establish quantitative relationships between potential critical parameters (related to e.g. product, process, method) and a defined response (e.g. product quality).
Compared to other industries, the (bio)pharmaceutical industry has been a relatively late adopter of DoE. Nowadays, DoE has become a recognized tool likely boosted by the implementation of Quality by Design (QbD), the approach to design a quality product and a well understood, controlled and high performing manufacturing process.
In the experience of Venn life Sciences scientists in Big pharma are well aware of the value of DoE in both early and late stages of development. DoE can support formulation development and serves to optimize and establish an optimal and robust set of operating conditions for manufacturing processes and analytical test methods. Scientists in Big pharma are often in the privileged position to have support from a statistical department which can help in the design of the experiments and result evaluation supported by advanced (costly) software, thereby lowering to barrier to use DoE.
For small and midsize (bio)pharma companies as well as CMOs, there is often some hesitation to use DoE. Various reasons for that may be given. One is that scientists are lacking the appropriate expertise to use DoE, often in combination with not having access to software programs to create designs and perform statistical evaluations. Stat-Ease, Minitab or JMP are examples of user-friendly software programs offering the most common experimental designs. Another reason for the slower adoption of DoE lies in the fact that smaller (bio)pharma companies and CMOs are often struggling when to best adopt DoE, either in early product -, process- or test method development or in later stage development. In our experience we often see that companies in early development perform too many (costly) experiments, often not in a very well-structured manner. In addition, in many cases the traditional one-factor-at-a-time (OFAT) approach is used which does not provide insight in potential interaction between factors. As a possible consequence follow-up studies may not confirm earlier experiments, or a process or analytical method is not as robust as anticipated.
The application of DoE is highly beneficial for companies working at early and late stage (bio)pharmaceutical product, process and method development. The adoption of DoE will short track your development, saving precious resources and costs. Give it a try!

Gerben Wynia (MSc) is senior CMC consultant at Venn Life Sciences. He has over 25 years of experience in pharmaceutical R&D and has worked on the development of both small molecules and biomolecules in early and late stages of product development. He has expertise in the use of statistical methodology in the development of products, manufacturing processes and analytical methods.
If you are interested to learn more about how DoE can support your development activities, please contact Venn life Sciences at getintouch@vennlife.com. We would welcome the opportunity to discuss how DoE might be of benefit to your company’s development. Venn life sciences offers help in risk assessments, the set-up of DoE experiments, and statistical evaluation and interpretation of results.
Data from therapy development, especially those involving cells, can quickly mushroom into an unmanageable pile in the lab — what happens when you move to the clinic? We sat down with Venn Life Sciences to discuss effective strategies in data management that might benefit companies looking to bring a therapy like Tregs to market.
Cell therapies are one of the major trends in biotech, and they’re being investigated as a therapy for everything from cancer to chronic inflammation. Say you’re a company looking to leverage T-cells, the ‘soldiers of the immune
If all goes well, you might succeed in finding a cure in the lab, but what happens when you scale up for clinical trials? From their PhD work, scientists are trained to manage laboratory-scale datasets, but the industrial expansion could pose a daunting challenge, especially when it comes to cell therapies.
As an example, Christian Le Bras, Head of Venn Life Science’s Interactive Response Technology department, walked us through the process of developing a therapy from Tregs, regulatory T-cells that modulate an immune response, and the management of the accompanying clinical data.
To start, each patient’s own blood cells must be collected, processed and prepared. In the lab, you might deal with one or a few; but in the clinic, you’d have to manage thousands. Once blood samples are collected, they have to be purified to obtain the Tregs. These are then “educated” ex-vivo by stimulation with antigens so that they are able to recognise them when they are re-introduced to the body. These choice Tregs are then isolated and scaled up for industrial expansion.
Mass production is where the data gets messy in a hurry: to effectively run clinical trials, enough Tregs have to be produced to cover multiple doses administered over several years by IV injections. To whom do you give which dose and when? Is the material even good anymore? An effective data management system can keep all of this organized for you.
With all of your doses squared away, you also have to make sure they don’t degrade. Cells are fragile and have to be kept under tightly controlled conditions. With this in mind, Venn Life Sciences developed a technology to record and track blood samples as well as target cell density, so a clinician can keep tabs on available treatments.
Once you’re sure of the quality of your samples, you can start treating patients. In Treg therapies, the cells will use the antigens for which they were tuned as a beacon to home in on the inflammation afflicting the patients. The antigens then activate the cells to proliferate and release immune suppressive factors to quell the inflammatory response. Once it has been treated, the Tregs are rapidly cleared.
Sounds like a simple step, right? Perhaps, but the process becomes dramatically more complex when you add the necessary double blinding methodology to guard against bias! Venn’s IRT makes this easier with its randomly assignment function: the software implements complex stratification to match patients to treatment arms with minimal bias and the option of subpopulation analysis.
The sheer bulk of this data might seem cumbersome, but it doesn’t have to be. Venn designed IRT to be portable so a clinical researcher can access the database on any numbers, update results or randomize the treatment group of a patient. In fact, Christian says the company’s goal is to make it increasingly automated to require less effort in the management of patient use and drug logistics.
While this technology might sound like icing on the cake, Christian warned us not to underestimate its importance. Even if you have stunning results, they won’t be useful if you can’t find them or spend an inordinate amount of time searching for them. Time is of the essence in bringing a competitive therapy through the clinic and to market: saving a few months can translate to millions of euros in extra revenue. With such tools, we do have the capability of organizing adaptive design that could accelerate the obtainment of positive results.
With this in mind, Venn Life Sciences aims to expedite clinical development by addressing the challenges of tracking samples and results on the huge scale of such trials. Accordingly, the company has designed IRT to provide a streamlined and quality driven method to manage your data in a more time and cost-effective way.
Want to know more about IRT? Give them a call on +3340211970, or drop Venn Life Sciences a line!
By using smart designs, sponsors can save time and money while optimizing the knowledge accrued during the development of their products.
“Traditional” study designs and statistical methodology do not allow changing the main features of a study protocol once the study has been initiated. The use of interim analyses, and in particularly the development of group sequential analysis methods introduces some flexibility in the study designs by allowing interruption of treatment arms (or the whole study) after early demonstration of efficacy (or evidence of futility) at a planned interim analysis.
Advances in the methodology of clinical studies provide even more flexibility by allowing the modification of some features of the design based on information collected during interim analyses. Such a setup is known as an adaptive study design, or among other designations a flexible, smart or multistage design.
In its 2004 Innovation/Stagnation: Challenge and Opportunity on the Critical Path to New Medical Product report, the US Food and Drug Administration (FDA) identified an urgent need to improve the efficiency and effectiveness of the clinical trial process, including trial design, endpoints, and analyses, and stated that in many cases, developers have no choice but to use the tools and concepts of the last century to assess this century’s candidates[1]. In the following Critical Path Initiative Opportunities List (March 2006), adaptive designs are identified as one way to streamline Clinical Trials[2]. The Regulatory bodies have issued guidance documents on the topic of adaptive designs. The EMA document was adopted in 2007[3] while the FDA draft guidance was issued in 2010[4].
The methodology experts at Venn Life Sciences have risen to the challenge by providing expertise and experience in helping clients to design such adaptive clinical studies.
So what is an adaptive design and how does it work?
An adaptive study design uses accumulating data to decide how to modify aspects of the study as it continues, without undermining the validity and integrity of the trial (Gallo et al. 2006[5]). The design can be changed only if the modifications were prospectively planned and if sound procedures and statistical methods are employed that guarantee the control of operational bias and the correctness of statistical inferences.
What are the pros and cons?
Beyond the practical advantages (faster, more efficient, and cheaper drug development) to such a “smart” design, the setup also has an ethical upside. Fewer patients are exposed to ineffective or harmful treatments and fewer patients are needed for the clinical development programs. From a business perspective, the more efficient clinical development process means that better decisions are made, resources are used more effectively and the final product is registered faster.
Designing an adaptive study sounds great, but it isn’t simple. The adaptations have to account for correct statistical inference, consistency between stages, minimized operational bias to avoid impacting the validity; further, integrity must be preserved by performing only pre-planned adaptations and by maintaining the blind to ensure that convincing results will be provided to the scientific community.
Moreover, an adaptive design requires rigorous planning and more complex statistical methods. It is undeniably an inherently complex procedure because of the need of interim analyses, independent data monitoring committee (DMC), independent statistical centre, and possible difficulties in regulatory buy-in.
What is the future of adaptive designs?
All these hurdles result in only a few adaptive designs being conducted. In a recent review, Hatfield et al [6] observed that the use of adaptive designs has increased from 11/10000 registered trials over the 2001-2005 period to 38/10000 over 2012-2013. The authors conclude that this rate remains low and that there may be disease areas in which ADs are being underutilised. We should expect more adaptive design trials to be conducted in the future, particularly in the early phases (exploratory trials) of clinical development.
Venn Life Sciences is up to the task of tackling the methodological and operational hurdles of adaptive designs for their clients. After the design phase is complete and the proposed study or development plan has been discussed with and approved by the regulatory authorities, Venn’s teams can implement it in the clinic to achieve everything from regulatory approvals and site selection to final statistical analysis and clinical study report writing. The company uses adequate logistical platforms, like interactive randomization technologies, electronic data capture, and a network of independent statisticians to prepare interim analyses for DMCs.
These powerful capabilities make it possible for Venn to implement and conduct successful adaptive clinical trials.
For further information please contact Venn France on +33 1 402 11 970 or check out www.vennlifesciences.com